Abstract
Introduction: Acute promyelocytic leukemia (APL) is defined by unique morphological features, typically abnormal promyelocytes containing azurophilic granules. Genomic basis of APL is characterized by the translocation t(15;17)(q22;q21), inducing fusions between promyelocytic leukemia (PML) and retinoic acid receptor-a (RARA). APL cells with PML-RARA respond to all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), and recent clinical trials have achieved excellent outcome for typical APL with PML-RARA . Although a certain fraction of APL cases lacks PMR-RARA, most of them had translocation including RARA and other genes, namely, PLZF, NPM, and TBL1XR1 . Eventually, all known APL-associated translocations involve RARA, accounting for 99% of APL. However, RARA rearrangement is unable to be detected in the rest of APL cases, and molecular mechanisms underlying this small subset is still unclear. We here analyzed APL cases without RARA translocation, and identified a novel RARB fusion in the majority of patients with RARA -negative APL.
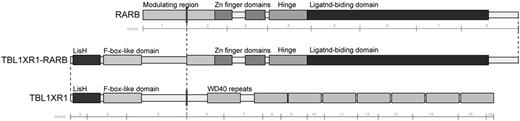
Materials and methods: We first performed whole-genome sequencing to identify potential genomic alterations in a case with RARA -negative APL, and successfully identified an in-frame fusion between TBL1XR1 and RARB . As RARB, encodes retinoic acid receptor-b, and RARA are both members of the nuclear receptor superfamily, we further searched for RARB alterations in additional 4 cases diagnosed APL morphologically lacking RARA translocation, using whole-transcriptome sequencing, RT-PCR, and FISH, according to sample availability. The five RARA -negative APL cases consist of one male and four females, and the median age of the cases were 3 years (range, 2-4 years of age). All cases were refractory to ATRA, and multiagent chemotherapy was given as acute myeloblastic leukemia but four cases suffered relapse or failed to achieve remission. To investigate functional effect of this novel fusion on leukemogenesis, we then transduced cell lines, bone marrow cells of mice, and human cord blood with TBL1XR1-RARB, RARB, and PML-RARA . Influence of all-trans retinoic acid (ATRA), arsenic trioxide (ATO) was also assessed.
Results: Four of the five (80%) cases had RARB translocations (three had the TBL1XR1-RARB fusion (Figure), and one had RARB rearrangement detected by FISH although partner gene of the case could not be determined). The novel fusion protein, TBL1XR1-RARB, could homodimerize, and diminished transcriptional activity with a dominant negative effect on both RARA and RARB. Transduced U937 cell lines with TBL1XR1-RARB showed cell differentiation with increased expression of CD11b when treated with ATRA, and neutrophil differentiation was confirmed by morphology. These effect of TBL1XR1-RARB were cancelled by administration of ATRA, although the impact was partial compared to that of PML-RARA. ATO, leading PML-RARA degradation by biding PML, could not affect TBL1XR1-RARB transduced cell lines. Colony replating assay demonstrated that this TBL1XR1-RARB could enhance replating ability of murine bone marrow. Differentiation of human cord blood was blocked by TBL1XR1-RARB, as well as PML-RAR, which was also confirmed by morphological features.
Conclusion: We found that the majority of RARA-negative APL had RARB translocation, defining a novel genetic subgroup in APL. Unlike wild type RARB, the TBL1XR1-RARB proteins homodimerize, suppress retinoic acid pathway, and are confirmed as having oncogenic potency, as well as PML-RARA. Our study confirmed that dysregulation in retinoic acid pathway was a hallmark of APL, however, response to ATRA was partial, which is concordant to clinical resistance to ATRA of RARA -negative APL. Thus, in the context of precision medicine, RARB alteration should be screened for RARA -negative APL cases.
Kitamura: Daiichi Sankyo: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal